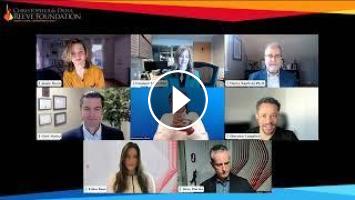
Christopher & Dana Reeve Foundation's panel discussion about the recent FDA market authorization for the ONWARD ARC-EX System in the US. ARC-EX is the first and only FDA approved technology shown to improve hand strength and sensation after chronic spinal cord injury (SCI).
Here are some key highlights of the ARC-EX System from the Up-LIFT clinical trial1:
• 90% of clinical trial participants showed improved strength or function in clinical trials
• Benefits seen up to 34 years post-injury in clinical trials
• Named a TIME Magazine Best Invention of 2024
Dr. Marco Baptista hosted a panel that included Up-LIFT principal investigators Drs. Chet Moritz and Candace Tefertiller, trial participants Sherown Campbell and Jessie Owen, and ONWARD Medical CEO Dave Marver and VP Clinical, Regulatory, and Quality Erika Ross Ellison.
The ARC-EX System is authorized only for professional use in the United States. ONWARD Medical will begin a limited release of the system in select rehabilitation centers starting in January 2025, with nationwide availability anticipated by April 2025. This phased approach is intended to ensure a safe and seamless integration of the new ARC-EX System into daily clinical practice.
For any inquiries or more information, please complete this form: https://survey.onwd.com/support. Connect with ONWARD via social media @onwdempowered.
ARC-EX Indication for Use (US): The ARC-EX System is intended to deliver programmed, transcutaneous electrical spinal cord stimulation in conjunction with functional task practice in the clinic to improve hand sensation and strength in individuals between 18 and 75 years old that present with a chronic, non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive).
Trademarks: ONWARD, ARC-EX, and the stylized O-Logo are proprietary and registered trademarks of ONWARD Medical. Unauthorized use is strictly prohibited.
1Moritz, Chet, et al. “Non-invasive spinal cord stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial.” Nature Medicine. 2024.
Here are some key highlights of the ARC-EX System from the Up-LIFT clinical trial1:
• 90% of clinical trial participants showed improved strength or function in clinical trials
• Benefits seen up to 34 years post-injury in clinical trials
• Named a TIME Magazine Best Invention of 2024
Dr. Marco Baptista hosted a panel that included Up-LIFT principal investigators Drs. Chet Moritz and Candace Tefertiller, trial participants Sherown Campbell and Jessie Owen, and ONWARD Medical CEO Dave Marver and VP Clinical, Regulatory, and Quality Erika Ross Ellison.
The ARC-EX System is authorized only for professional use in the United States. ONWARD Medical will begin a limited release of the system in select rehabilitation centers starting in January 2025, with nationwide availability anticipated by April 2025. This phased approach is intended to ensure a safe and seamless integration of the new ARC-EX System into daily clinical practice.
For any inquiries or more information, please complete this form: https://survey.onwd.com/support. Connect with ONWARD via social media @onwdempowered.
ARC-EX Indication for Use (US): The ARC-EX System is intended to deliver programmed, transcutaneous electrical spinal cord stimulation in conjunction with functional task practice in the clinic to improve hand sensation and strength in individuals between 18 and 75 years old that present with a chronic, non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive).
Trademarks: ONWARD, ARC-EX, and the stylized O-Logo are proprietary and registered trademarks of ONWARD Medical. Unauthorized use is strictly prohibited.
1Moritz, Chet, et al. “Non-invasive spinal cord stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial.” Nature Medicine. 2024.
- Catégories
- Webinar
- Mots-clés
- paralyzed, reeve foundation












Commentaires